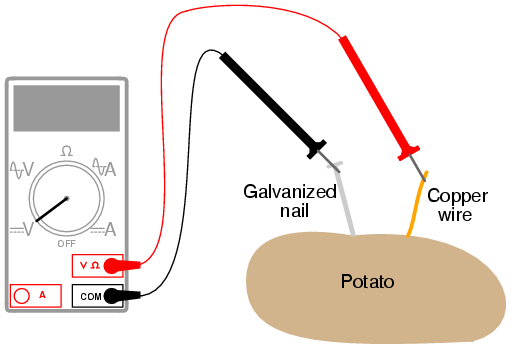
1.Build a Potato battery
PARTS AND MATERIALS
- One large potato
- (or) One lemon (optional)
- Strip of zinc, or galvanized metal
- Piece of thick copper wire
The correct terminology is potato cell. A cell is a single electro-chemical unit, while a battery is a group of two or more cells.
For the zinc electrode, a large galvanized nail works well. Nails with a thick, rough zinc texture are preferable to galvanized nails that are smooth. Note: in the process the zinc or iron will be dissolved/corroded. If one uses sandpaper, etc. to clean the zinc the process will work better.
Another combination not mentioned is the use of saltwater, copper sulfate, dilute sulfuric (battery) acid, or vinegar in a glass. Be careful of all of these materials.
video link
2.Build a lemon battery
Has your flashlight ever stopped working because the batteries were dead? It’s no fun walking around in complete darkness. Batteries are everywhere—in our toys, in our cars, in our flashlights and cell phones. But how do they work? What makes them stop working? You can learn how to make a lemon battery to learn more about these very important devices.Problem:
How does a battery work?
Materials:
- A lemon, or other citrus fruit
- 18 (or smaller) gauge copper wire
- Wire stripper/clipper
- A grown-up or older friend
- Steel paper clip, small galvanized nail (one that is covered in zinc), or a piece of zinc (ideal)
procedure
- Ask your grown-up to use the wire strippers to first strip about 2 1/2 inches of plastic insulation off the copper wire. Then, request that the grown-up clip that piece of stripped wire off of the main roll.
- Carefully straighten the steel paper clip. Use the wire clippers to cut it to the same length as your copper wire.
- Use the sandpaper to rub out any rough spots in your wire or paperclip. You are going to be touching the wire ends to your tongue, so you want them to be smooth. If you are using the zinc covered nail or piece, scratch it lightly with the sand paper to expose a fresh surface.
- Roll the lemon gently on a table to break the cell walls and loosen up the juice inside. The sour juice is needed for the chemical reaction that you are about to start. The fact that the juice is sour should give us some hints about what kind of chemicals make up lemon juice. What do you think the sour flavor might tell us?
- Carefully stick the copper wire about 1 inch into the lemon.
- Make sure your tongue is moist with saliva, or spit. Touch your tongue to the copper wire. Do you notice anything?
- Stick the paperclip, zinc covered nail or zinc strip into a spot in the lemon about 1/4 inch away from the copper wire. Make sure the wires don’t touch. The wires need to be close to each other because they will be swapping matter in the chemical reaction. If they are too far apart, the matter might lose their way.
- This time, touch your moistened tongue to both wire ends. What do you notice?

Results
When you touched your tongue to just the copper wire, you most likely would not have noticed anything unusual. When you touched your tongue to BOTH of the metal ends, you might have felt a tingle, or noticed a metallic taste.
Why
The tingle or metal taste you noticed shows that your lemon battery was generating anelectric current. That means tiny electrons were moving across the surface of your tongue. Electrons are subatomic particles that zoom around an atom’s center and make up the part of the atom that is negatively charged.
The lemon battery you made is a type of battery called a voltaic battery. These types of batteries are made of two different metals, which act as electrodes, or places where electrons can enter or leave a battery. In your case, the electrical current entered your tongue, which is why you felt a tingle.
So why were we able to stick electrodes into a lemon and get a battery? All voltaic batteries need their metals to be placed in an electrolyte. An electrolyte is a substance that can carry electrical current when dissolved in water. The tiny bit of salt in your saliva makes your saliva an electrolyte, and the sour citric acid does the same thing for lemon juice. Batteries stop working when there is not enough of the electrolyte to react with the metal or not enough metal left to react with the electrolyte.
video link
3.Build a saltwater battery
video link


No comments:
Post a Comment